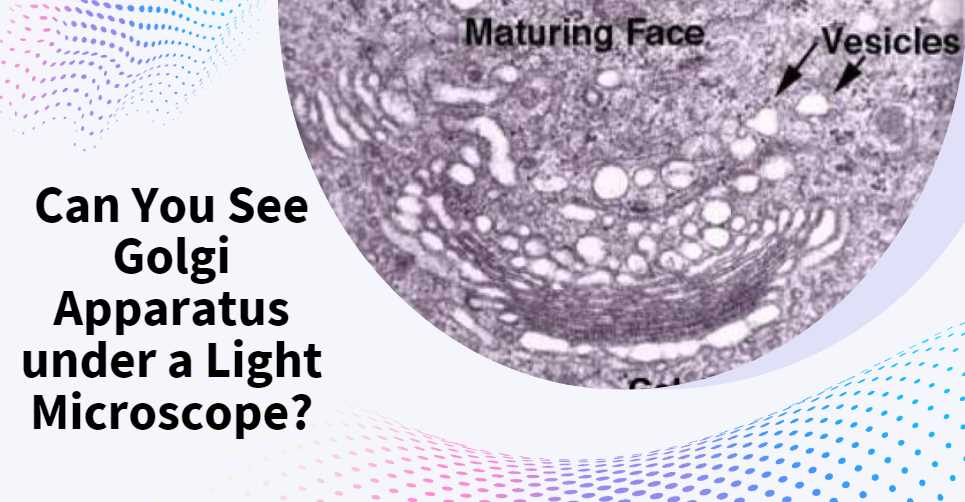
How to Turn On a Microscope: A Step-by-Step Guide for Beginners
To turn on a microscope, ensure that the power switch is set to the “on” position. This is typically located on the base or side of the microscope. Next, adjust the light intensity using the brightness control to your preference. Step Duration (Approx.) Key Considerations Common Mistakes Power On 1-2 seconds Ensure proper power source […]
How to Turn On a Microscope: A Step-by-Step Guide for Beginners Read More »